Welcome to the November edition of IslandWatch! We're excited to kick off this issue with a milestone announcement regarding our Phase 2a/b clinical trial. In the last week of November, we announced that our lead candidate, ISLA-101, has shown safety and anti-dengue activity in the Phase 2a cohort of our PROTECT Phase 2a/b trial. This significant step forward was featured in The Australian, spotlighting Island’s innovative efforts to combat dengue with our drug candidate ISLA-101, which is being trialled as both a prophylactic and therapeutic solution to this challenging disease.
Other highlights and activities from the month of November included an investor roadshow in select cities around Australia, the company’s 2024 AGM, and some changes to Island’s board, including the appointment of Mr Phillip Lynch as Executive Chair. Please read on for further details on these developments.
As dengue fever continues to pose a global health challenge, this month, the news headlines reminded us again the widespread impact of this mosquito-borne disease. In many parts of Asia, the rainy season has rolled in, increasing the risk of mosquito outbreaks! Indonesian broadcaster VOI highlighted the critical role of community participation in preventing dengue outbreaks, advocating for active measures like eliminating stagnant water, using mosquito repellents, and maintaining clean surroundings.
With dengue infecting 400 million people annually, Island Pharmaceuticals remains steadfast in our mission to tackle this growing global health threat. Read on for more updates on recent outbreaks and company insights shaping the future of dengue prevention and treatment.
Dengue Live Map, as of 2 Dec 2024
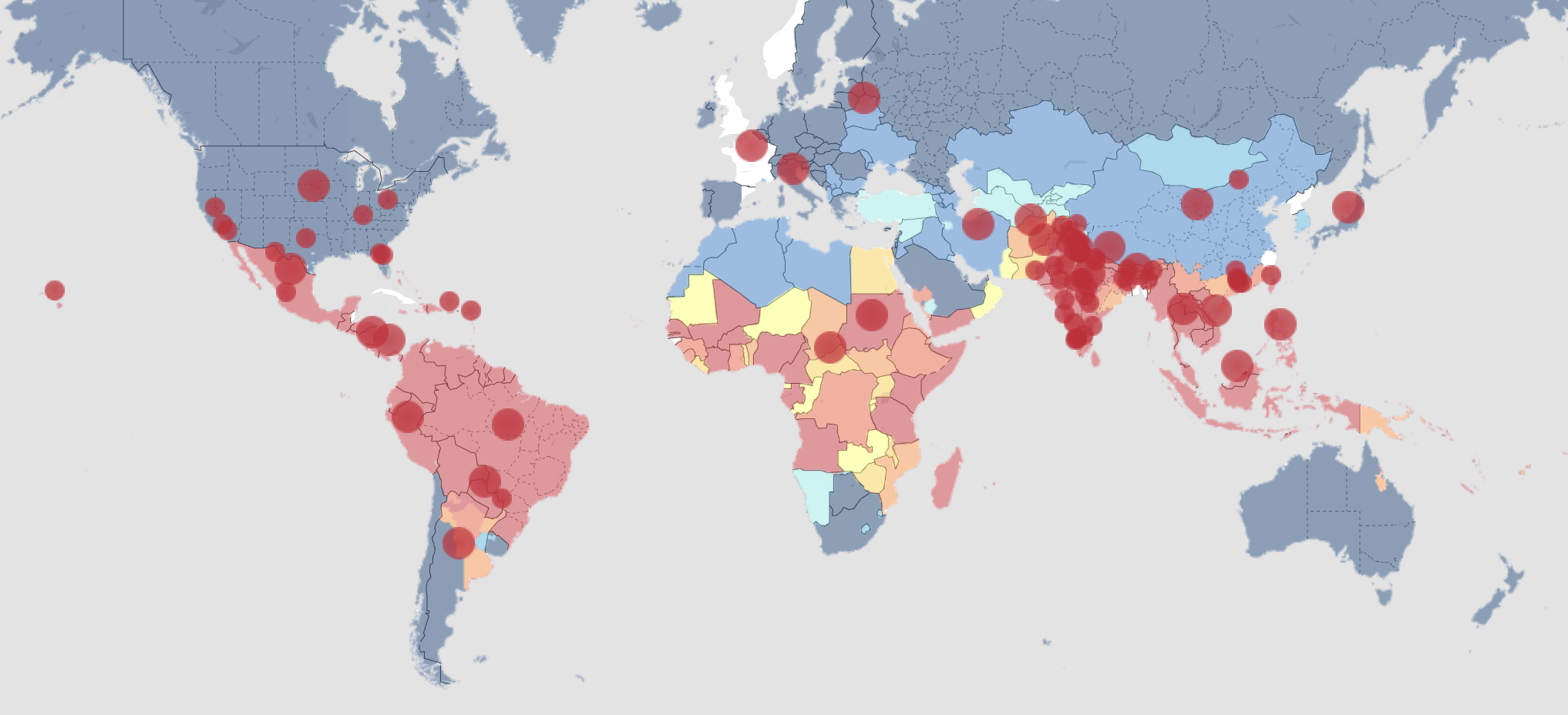
Credit (click to check live dengue status): https://www.healthmap.org/dengue/en/
HEADLINES OF THE MONTH
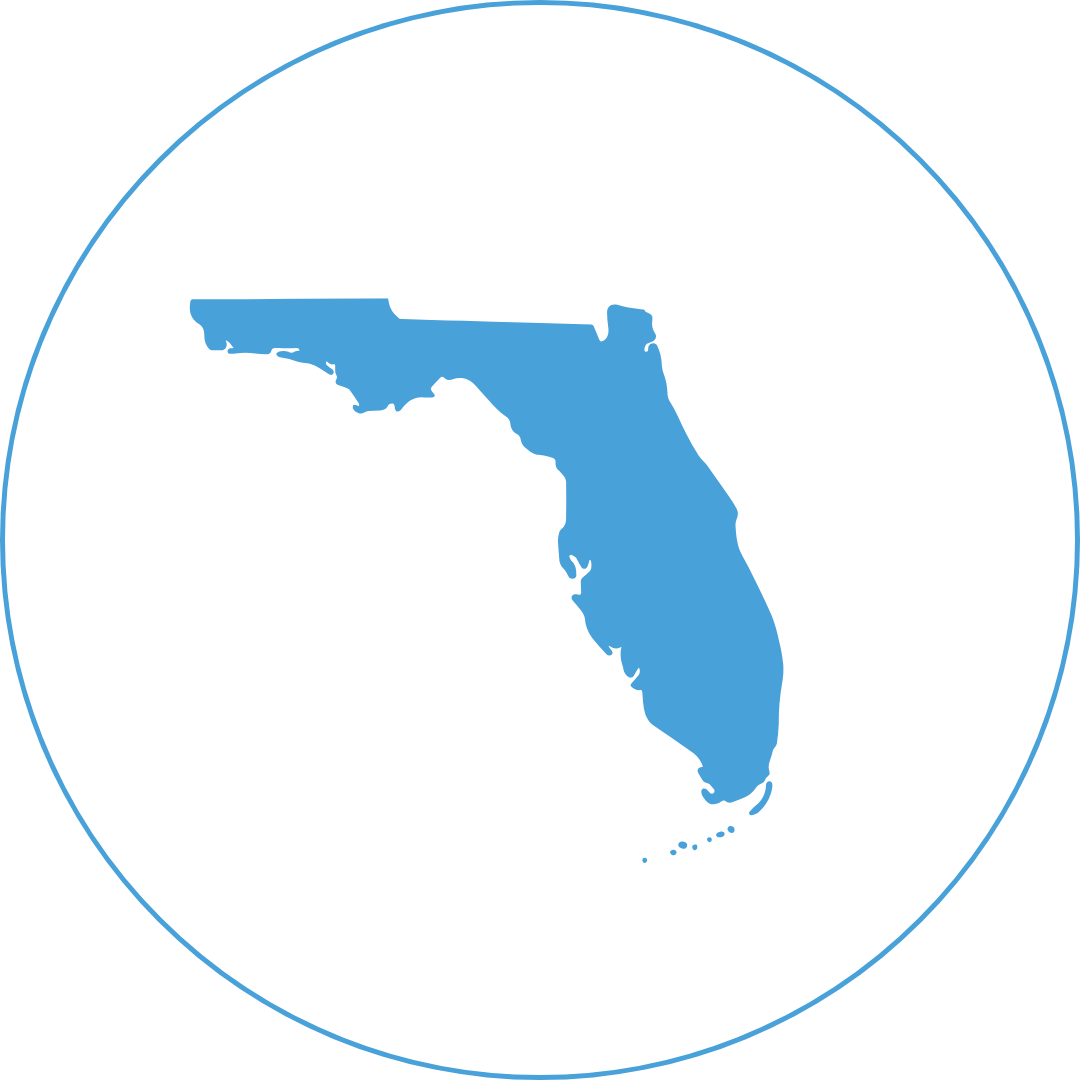 |
Florida’s Dengue Spike After Hurricane Milton
Dengue cases rise in Florida's Tampa Bay after Hurricane Milton, as standing water fuels mosquito breeding. Officials ramp up control measures.
Read more
|
 |
Bangladesh Battles Worst Dengue Outbreak
Bangladesh faces its deadliest dengue crisis, with over 400 deaths and 78,000 hospitalisations. Rising temps and heavy monsoons worsen the outbreak.
Read more
|
 |
Dengue Surge in Italy’s Marche Region
Italian scientists are sounding an alarm over a surge in cases of dengue fever cases in the, as high temperatures have encouraged the spread of the virus.
Read more
|
LATEST ISLAND PHARMACEUTICAL UPDATES
November was an eventful month for Island, which included some significant updates on the ISLA-101 Phase 2a/b clinical trial in dengue fever. Read on for more details.
Phase 2a/b PROTECT study update
As mentioned earlier, Island announced that ISLA-101 has shown safety and anti-dengue activity in the Phase 2a cohort of the PROTECT Phase 2a/b trial. The Safety Review Committee (SRC) further noted that blood levels of ISLA-101 were seen as expected, and given positive safety and efficacy signals, recommending that Island proceed with the therapeutic Phase 2b cohort.
Read the full announcement >>
Watch an interview with Island CEO >>
In an online investor briefing session, Island CEO and Managing Director Dr David Foster discussed the positive Safety Review Committee findings from the Phase 2a cohort and outlined next steps for the company. The presentation was followed by a Q&A session with investors.
Watch full recording for the session >>
Non-deal roadshows
During the month of November, Dr Foster visited Australia for a series of non-deal roadshows. Dr Foster met with investors, brokers and analysts in Sydney, Perth and Melbourne where he provided updates on the ISLA-101 Phase 2a/b PROTECT clinical trial, discussed the galidesivir due diligence program, and covered other recent developments with the company.
While in Australia, Dr Foster was also invited to present at the Twilight Investor Briefing in Melbourne and the Stock Soiree investor event, hosted by Market Open in Perth.
Watch a recording of Dr Foster’s presentation at Twilight Investor Briefing (starts at 20’06’’) >>
Board changes
Dr Paul MacLeman and Dr Anna Lavelle retired from the Island Board following the Company’s 2024 Annual General Meeting. Mr Phillip Lynch was appointed Executive Chairman, with effect immediately after the 2024 Annual General Meeting. Island would like to thank Dr MacLeman and Dr Lavelle for their immense contributions to the company’s growth and evolution, and extend a warm welcome to Mr Lynch, who will bring invaluable knowledge and expertise to our company.

Island welcomes Mr Phillip Lynch to the company Board as Executive Chairman
Island’s 2024 AGM
Island held its 2024 Annual General Meeting on Tuesday, 19 November 2024 at 9:00 am (AEDT). The resolutions were voted in accordance with the Notice of Annual General Meeting previously advised to the Australian Securities Exchange.
Results of the AGM can be accessed here >>
Watch a recording of CEO presentation at the AGM >>
Feature in The Australian
As seen in The Australian, Island was featured in Tim Boreham’s column this month. The article highlighted how ISLA-101 addresses a significant market gap, given there is currently no treatment for dengue fever and very limited vaccine options on the market.
Read more >>
DID YOU KNOW?
In Brazil, a novel approach to combat mosquitoes involves releasing genetically modified Aedes aegypti mosquitoes. These modified mosquitoes are designed to produce offspring that die before reaching adulthood, effectively reducing the population of mosquitoes by up to 90% in targeted areas. There have been concerns that these genetically modified mosquitoes sometimes produce viable offspring that can survive but that is still yet to be determined.
.png)
