About ISLA-101
ISLA-101 is our lead asset which is being repurposed for the prevention and treatment of dengue fever and other mosquito (or vector) borne diseases.
In its former life, ISLA-101 was the subject of 48 Phase I and II human clinical trials - work which saw it verified as safe in humans by multiple regulators. While unsuccessful in its original planned uses as either cancer or respiratory therapeutics, pre-clinical work conducted at Monash University in rodent and human models has demonstrated ISLA-101 to be extremely promising as an antiviral drug.
Mechanism of action
A conserved feature of the mosquito borne viruses targeted by ISLA-101 is the requirement that a particular viral protein enter the host cell nucleus. Based on the studies at Monash University, it has been demonstrated that ISLA-101 prevents nuclear entry of this protein, preventing propagation of the viral infection. In addition, studies from Monash University and Harvard University demonstrate that this activity is protective in animal models of dengue infection and Zika infection, respectively. As such, ISLA-101 is designed to act as an inhibitor to target the stages and proteins of Flaviviruses, as shown in the illustration below.
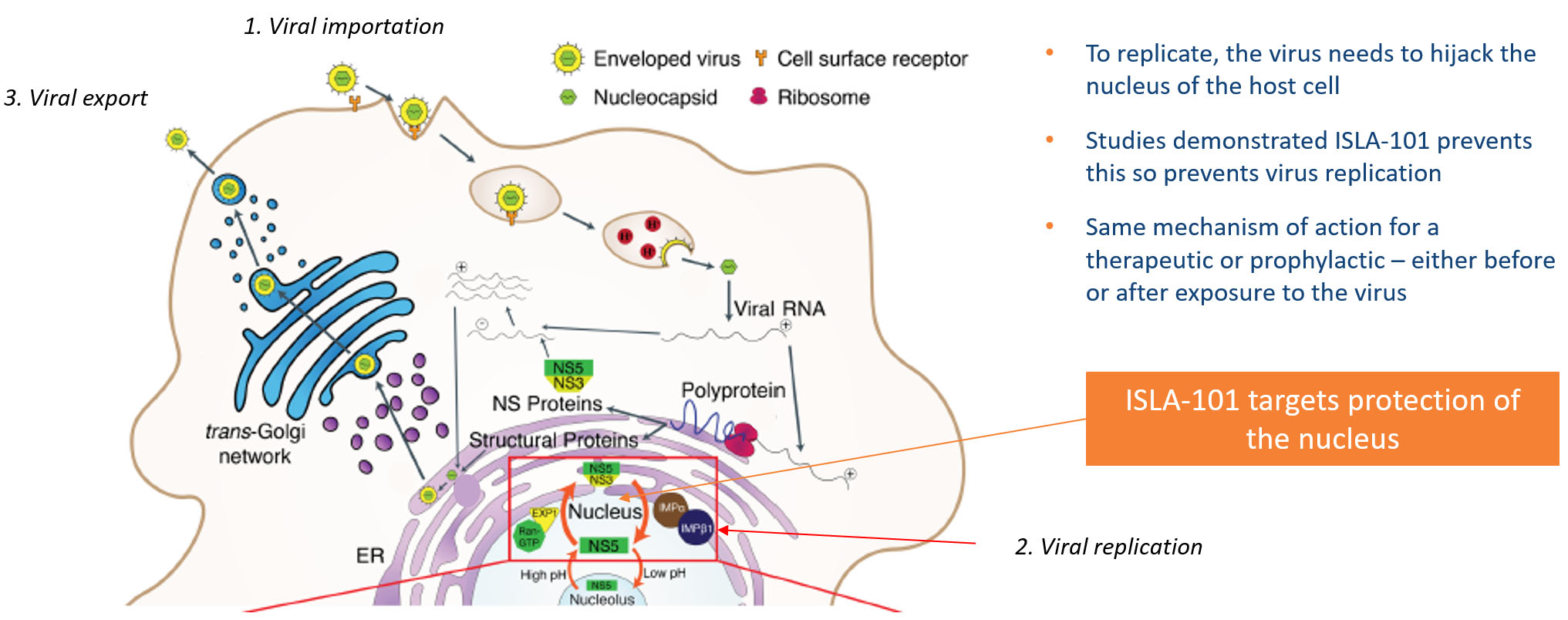
Island is progressing ISLA-101 into clinical trials in dengue infected subjects.
The drug also has the potential to be used to prevent or treat a number of viruses including dengue, Zika West Nile and Yellow Fever. It could potentially displace vaccines.
Assuming ISLA-101 is given FDA approval, Island may be eligible to obtain a "Priority Review Voucher" at the time of approval. This means that as well as getting approval to commercialise ISLA-101, the Priority Review Voucher (PRV) will permit Island to expedite the FDA approval process for a new drug or sell the PRV to a third party. PRVs have recently sold for at or slightly above US$110m.
While ISLA-101 is an oral formulation, the Company is also considering alternative formulations, including a long-acting oral formulation and intravenous formulations for severe dengue infections.
.png)
